Bioarchaeology International
Volume 6, Numbers 1–2: 133–148
DOI: 10.5744/bi.2021.0002
Received 17 January 2021
Revised 31 March 2021
Accepted 10 April 2021
Human-Animal Interactions and Infectious Disease: A View for Bioarchaeology
Judith Littletona*, Sarah Karstensa, Mark Bussea, and Nicholas Malonea
ABSTRACT Zoonoses are significant in human histories, and in histories of other species and the environment. Diseases have been an important evolutionary force, not just the major epidemics but the quieter endemic diseases. These infectious diseases comprise complex events and cycles involving multiple actors (humans, animals, and microorganisms). Despite difficulties of preservation, identification, and interpretation, bioarchaeologists have often analyzed zoonotic diseases. However, these studies have tended to focus on an individual disease and its emergence as opposed to the human-animal interactions and complex environmental cycles that underlie zoonotic disease more broadly. In this paper, after a brief review of zoonotic disease and bioarchaeological studies of it, we provide three contemporary case studies that point to the complexity of human-animal interaction and the socioecological circumstances involved in disease. We argue that adopting a One Health framework, which is based on Rudolf Virchow’s insight as well as approaches that emphasize time depth, multiple analytical scales, evolutionary understandings, and a consideration of human ideas and not just practices, would contribute to making bioarchaeology relevant to contemporary and future issues beyond the epidemiological transition model as modified by Barrett and Armelagos (Barrett et al. 1998; Barrett and Armelagos 2013).
Keywords: zoonosis; One Health; zooarchaeology; pandemics
Les zoonoses sont importantes dans l’histoire humaine, et en gros dans l’histoire d’autres espèces et de l’environnement. Les maladies ont été une force évolutive importante, non seulement les épidémies majeures, mais aussi les maladies endémiques les plus calmes. Ces maladies infectieuses comprennent des événements et des cycles complexes impliquant de multiples acteurs (humains, animaux et micro-organismes). Malgré les difficultés de préservation, d’identification et d’interprétation, les bioarchéologues ont souvent analysé les zoonoses. Cependant, ces études ont eu tendance à se concentrer sur la maladie individuelle et son émergence par opposition aux interactions homme-animal et aux cycles environnementaux complexes qui sous-tendent la maladie zoonotique de manière plus générale.
Dans cet article, après un bref examen de la maladie zoonotique et des études bioarchéologiques de celle-ci, nous fournissons trois études de cas contemporaines qui soulignent la complexité de l’interaction homme-animal et les circonstances socio-écologiques impliquées dans la maladie. Nous soutenons que l’adoption d’un cadre One Health basé sur la vision de Rudolf Virchow ainsi que sur des approches mettant l’accent sur la profondeur temps, les échelles analytiques multiples, les compréhensions évolutives et la prise en compte des idées humaines et non seulement des pratiques, contribuera à rendre la bioarchéologie pertinente pour les problèmes contemporains et futurs au-delà le modèle de transition épidémiologique tel que modifié par Barrett et Armelagos (Barrett et al. 1998; Barrett et Armelagos 2013).
Vocabulaire: zoonosis; One Health; zooarchéologie
Between animal and human medicine there is no dividing line—nor should there be. The object is different, but the experience obtained constitutes the basis of all medicine. (Rudolf Virchow [1821–1902], cited in Klauder [1958:170].)
It is commonly reported that around 60% of infectious diseases experienced by humans are zoonoses (i.e., pathogens shared with wild or domestic vertebrate animal populations) (Taylor et al. 2001). Worldwide attention in 2020 has been sharply focused on the possibility of new pathogens such as SARS-CoV-2. While COVID-19 is a zoonotic infection with high lethality for humans, the importance of zoonoses in human history is not only a result of a capacity to cause major mortality or morbidity in human populations. Some of the major infections of humans began as a zoonosis before evolving to be a primarily anthroponotic disease (e.g., HIV/AIDS), raising the question of what conditions promote such transitions (Wolfe et al. 2007). Other zoonoses (e.g., brucellosis), while causing low mortality in humans, have significant biological and social impacts, affecting livestock survival or meat and milk production (Bendrey and Fournié 2020). What can bioarchaeology add to our understanding of disease emergence, but also, what can the recognition of disease in the past add to our understanding of the past?
The epidemiological transition model, particularly as reworked by Armelagos and colleagues (Barrett et al. 1998; Barrett and Armelagos 2013), explicitly identifies the relationship between the emergence of zoonotic infectious disease and human history, postulating that the transition to agricultural practices and domestication increased opportunities for contact between humans and other animals, leading to a rise in infectious disease as a major cause of mortality and morbidity. A similar focus on sedentary habitation and close proximity was proposed by McNeill (1976). These models remain implicit or explicit in many treatments of zoonotic disease. Can bioarchaeological perspectives contribute more to this endeavor than what has become a truism?
Understanding zoonotic diseases, their emergence, evolution, and impact on human and animal populations is important in plotting long-term trajectories of disease, identifying drivers of disease emergence and persistence, and appreciating the interaction between human, animal, and environmental health, particularly drivers of resilience and buffers to exposure (Larsen 2018). We argue that integrative approaches (such as a One Health perspective that explicitly views environment, human, and animal health as linked [e.g., Waltner-Toews 2017]) and a more explicitly anthropological consideration of human-animal interactions (taking into account their biocultural complexity) may move us beyond the association of infectious disease with the agricultural transition and contribute to the place-specific deep time histories argued to be essential to understanding disease emergence (Wallace et al. 2015).
In this paper, we briefly review zoonotic transmission, survey recent bioarchaeological work (primarily zooarchaeological) on zoonoses, which is often very focused on a specific disease, and provide three case studies that demonstrate the complexity of human-animal–disease interactions and why an anthropological understanding of human practices and beliefs is a crucial part of unravelling history.
Types of Interactions between Humans, Animals, and Zoonoses
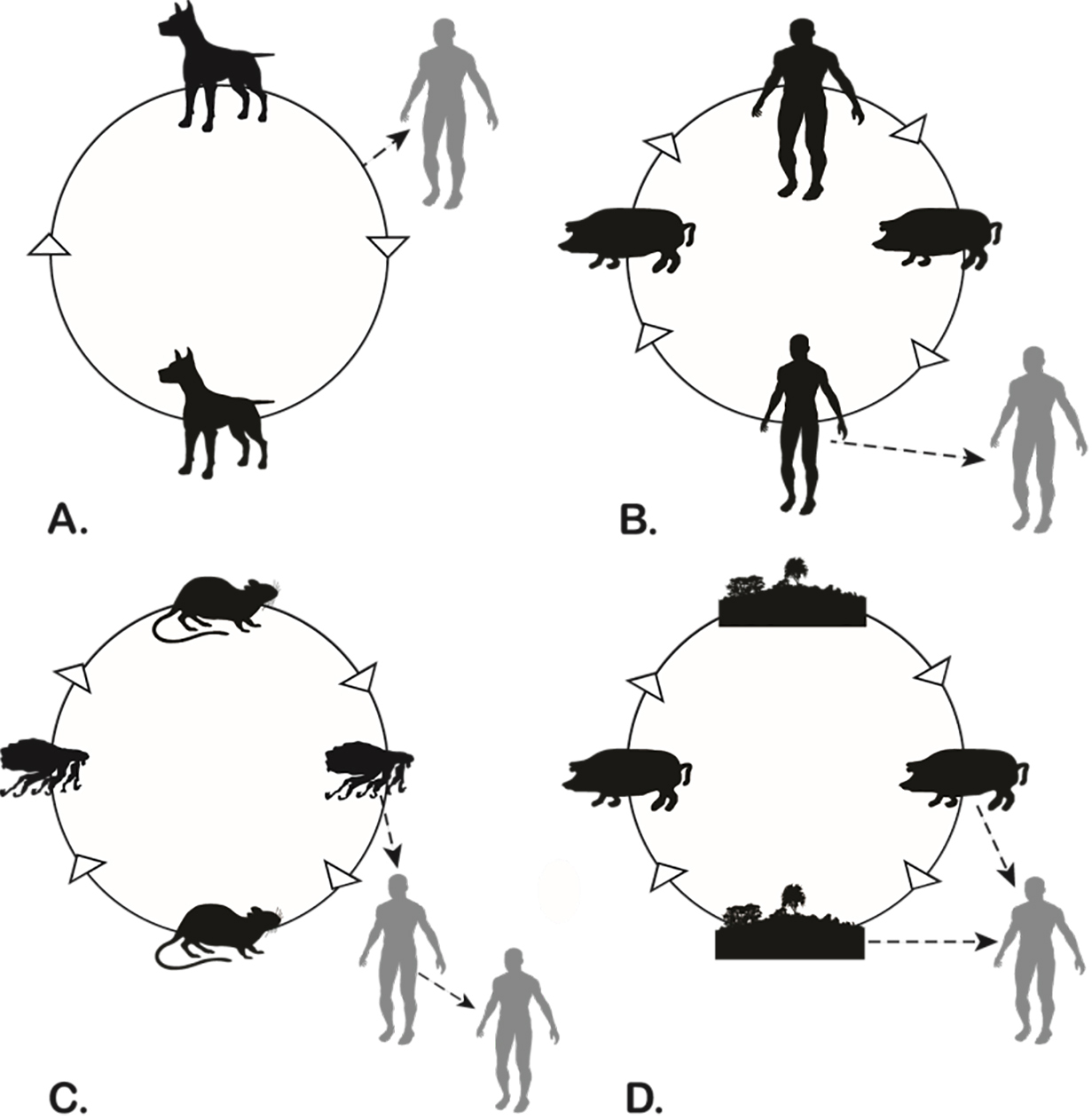
The multiple classifications of zoonoses highlight the complexity of these infections as a class. The term zoonosis itself was first coined by Rudolf Virchow in the latter part of the nineteenth century and includes anthropozoonosis (nonhuman animals to humans), zooanthroponosis (humans to nonhuman animals), and amphixenosis (nonhuman animals and humans are both reservoirs). A more meaningful classification is the maintenance cycle of the pathogen, which highlights the importance of the triad between host, pathogen, and environment (Chomel 2014). The maintenance cycles (shown in Fig. 1) are:
1. Direct zoonoses (orthozoonoses) (e.g., rabies);
2. Cyclozoonoses, which require for the developmental cycle of the agent more than one vertebrate species but no invertebrate host (e.g., human taeniasis or tapeworm infections where humans are the definitive hosts);
3. Pherozoonoses (or metazoonoses), which require both vertebrates and invertebrates for completion of the infectious cycle (e.g., plague, rickettsial infections, Lyme borreliosis); and
4. Saprozoonoses, which have a vertebrate host and an inanimate developmental site or reservoir (e.g., histoplasmosis).
Even in a direct zoonosis, the broader ecology of potential reservoirs and their relationships is crucial in disease transmission.
However, there are significant problems with analyzing past zoonotic infections, particularly when relying upon skeletal evidence. Not only is there the complex ecology of diseases at the human-animal interface but the reality of exploring those relationships from the evidence available in archaeological settings. Even in contemporary societies, many zoonoses are unrecognized or underdiagnosed: their symptoms in animals are nonspecific, they are often chronic, developing over several years, and they can co-occur. Many zoonoses are today classified as “neglected tropical diseases” (Maudlin et al. 2009) and a review of the bioarchaeological literature reflects the way in which some diseases are much more amenable to archaeological study than others.
Figure 1. The four types of zoonotic maintenance cycles: a. direct transmission (e.g., rabies); b. cyclozoonosis (e.g., taeniasis in humans or cysticercosis in pigs and humans); c. pherozoonosis (e.g., plague); d. saprozoonosis (e.g., ersipelothirx). Adapted from Chomel (2014:Figures 1–4).
Zoonotic Disease in Bioarchaeology
Bioarchaeological analyses of zoonotic diseases were traditionally limited to the study of those diseases that leave visible traces in the archaeological record, such as skeletal lesions or parasitic cysts. Only a small handful of zoonotic infections result in skeletal lesions that can be subject to traditional paleopathological analysis and those lesions are often nonspecific (e.g., cribra orbitalia as potentially indicative of malaria) or common to more than one infection (e.g., lesions on the spine resulting from Mycobacterium tuberculosis, M. bovis, or brucellosis). While these issues are common to osteological analyses of both human and nonhuman remains, taphonomic processes and biases in the archaeological record impact interpretation of disease in human and nonhuman species differently. Those zoonoses that result in skeletal lesions tend to be chronic conditions. In both humans and animals, this limits the number of identifiable diseases and leads to issues associated with the osteological paradox (see Wood et al. 1992). In addition, the variable lifespans, economic value, cultural significance, and levels of human investment in veterinary care across nonhuman species create additional biases because some animals (e.g., dogs, horses) tend to live to older ages and are therefore more likely to display skeletal pathologies associated with infectious disease (MacKinnon 2010). Unlike humans, the remains of animals in the archaeological record are rarely in the form of in situ burials; instead, assemblages of disarticulated, isolated bone fragments associated with food preparation create bias in the species and elements recovered and often make assessment of the distribution of pathological lesions within an individual animal impossible (Bartosiewicz 2013; Thomas 2017; Upex and Dobney 2011). It is, therefore, insufficient to look at indicators of disease in past human and animal remains in parallel as they find their way into the archaeological record for different reasons and have different life and depositional histories within particular, but only partially identifiable, social contexts. These additional taphonomic issues of animal remains and a lack of comparative veterinary data for many conditions mean that bioarchaeological studies of zoonoses have primarily relied on analysis of human remains and, therefore, the impacts of such diseases on human populations.
Many of the infections that result in skeletal lesions have been linked with the domestication of cattle, sheep, and goats. Given hypotheses of epidemiological transitions accompanying the adoption of agriculture (see Barrett et al. 1998; D’Anastasio et al. 2011; Fournié et al. 2017; Hershkovitz et al. 2008), many studies of the timing of the introduction and transmission pathways of zoonoses into human populations focus on domestication and the Neolithic transition. This is particularly true for the Mycobacterium tuberculosis complex and brucellosis. Brucellosis and infection with M. bovis are typically associated with the consumption of unpasteurized dairy products or prolonged contact with infected sheep, goats, or cattle and their bodily fluids (D’Anastasio et al. 2011; Moreno 2014; Murphy et al. 2009; Taylor et al. 2007; Wooding et al. 2019). M. tuberculosis was thought to have evolved from the M. bovis strain and then spread and been maintained in the denser sedentary populations of agricultural societies (Hershkovitz et al. 2008; Stead 1997; Stead et al. 1995). This hypothesis was supported by the identification of brucellosis and tuberculosis (TB) in human remains from sites with ethnohistoric and/or archaeological evidence for domestication of sheep, goats, and cattle and the consumption of dairy products (e.g., Brothwell 1965; Capasso 1999, 2002; Merrett 2004; Mutolo et al. 2012; Ortner and Frohlich 2007; Rashidi 2011).
TB and brucellosis have, however, also been identified through paleopathological and molecular analyses in the remains of hunter-gatherers in the Americas (Bos et al. 2014; Jones 2019), possibly in the Pacific (see McDonald et al. 2020), in predomestication settlements in Syria (Baker et al. 2015), and even in a late Pliocene specimen of Australopithecus africanus (D’Anastasio et al. 2009). The studies of Bos et al. (2014), Jones (2019), and McDonald et al. (2020) dispute suggestions that the origin of TB in the Americas and Pacific was commensurate with European contact.
One of the only examples of direct evidence from faunal remains of zoonoses or anthropozoonoses resulting from human-animal interactions is that of a case of TB in an Iroquoian dog from a sixteenth-century Iroquoian site in Ontario (Bathurst and Barta 2004). Archaeological and ethnohistoric evidence suggests that the role of dogs in Iroquoian society was a mixed one, with dogs being used in hunting and guardianship, as companion animals, and as food (Bathurst and Barta 2004). The consumption of dog meat, the scavenging and coprophagic habits of dogs probably in village middens, as well as the sharing of beds, food, and eating vessels with dogs would have created multiple potential pathways for the transmission of infection between humans and dogs (Bathurst and Barta 2004). This case demonstrates a range of potential transmission pathways for TB resulting from nonagricultural human-animal interactions.
As well as confirming the presence of TB infections in nonagricultural settings, in the past two decades, ancient DNA analysis and the sequencing of whole M. tuberculosis complex genomes have also forced a reconsideration of the evolutionary history of TB. Studies have suggested that M. tuberculosis likely existed and may well have been pathogenic to humans prior to the separation of the M. bovis lineage (Brosch et al. 2002; Zink et al. 2007). Ancient DNA analysis allows for the identification of zoonotic infections that do not result in diagnostic skeletal lesions. Chagas disease (Trypanosoma cruzi) is one such infection, identified in the tissues of mummies from South and Central America dating back as far as 9000 B.P. (Aufderheide et al. 2004; Fernandes et al. 2008; Solari 2011). The date of this disease and its distribution demonstrates how by encroaching and creating novel environments, humans altered the sylvatic life cycle of a disease, potentially promoting a switch from wild animals to humans as vectors (Aufderheide et al. 2004; Reinhard and Araújo 2015). Over 100 species of wild mammals act as reservoirs for Chagas disease, which is transmitted through several dozen insect species hiding in animals’ nests and lairs. Human residence in bat inhabited caves and the predation of wood rats may have brought humans into contact with the disease. However, a number of the insect vectors of the disease appear to have adapted to human dwellings, particularly the wattle and thatch huts built by early settlers, which provided ideal habitats for the nesting insects. The rearing of guinea pigs may also have provided a reservoir for the disease inside homes (Aufderheide et al. 2004; Coimbra 1988; Reinhard and Araújo 2015).
The examples of Chagas disease and the Iroquoian dog highlight the problematic nature of a dichotomous domesticate/wild classification for zoonotic vectors. Many species fall somewhere on a spectrum between these two extremes and their position within that spectrum and the nature of their interactions with humans varies across time and space. Modeling using a wider variety of both direct and indirect evidence can assist in better understanding the nuances of these human-animal–environment interactions that give rise to zoonotic infections and the responses to and outcomes of epidemics, particularly those that leave little or no visible trace in the archaeological record. The most common example of such modeling in bioarchaeology is the use of data from cemeteries associated with known historical epidemics to model the mortality and morbidity profiles of past epidemics and how people might have responded to such diseases. For example, DeWitte and Wood (2008) compared the age, sex, and morbidity profile of the East Smithfield cemetery in London, a cemetery used exclusively during the Black Death of 1349–1350, to other non-epidemic cemeteries and found that elderly people and those who had experienced physiological stress prior to the epidemic were at a greater risk of dying during the Black Death.
These models, however, focus primarily on the impact of disease on human populations. While an important contribution to our understanding of past and future epidemics, this is only one aspect of the story of any zoonotic epidemic. While still relatively few in number, examples of modeling incorporating zooarchaeological, geospatial, climate, ethnographic, and historical data alongside traditional bioarchaeological analyses offer an opportunity to create a fuller picture of the circumstances leading to and results of zoonotic outbreaks (e.g., Bendrey et al. 2019; Fournié et al. 2017; Gowland and Western 2012; Marciniak et al. 2018; Seetah et al. 2020; Smith-Guzmán et al. 2016). Gowland and Western (2012) provide an example of how modeling using multiple lines of evidence can overcome some of the limitations of traditional bioarchaeological analyses in their analysis of malaria in Anglo-Saxon England. Malaria is notoriously difficult to positively diagnose on the basis of skeletal lesions alone. While recent studies using clinical reference samples have attempted to associate a range of skeletal lesions with malarial infection (e.g., Smith-Guzmán 2015), cribra orbitalia remains the most commonly cited indicator of malaria in the bioarchaeological literature. Yet, malaria is only one of a number of possible underlying causes for cribra orbitalia. To determine the likely relationship between the presence of cribra orbitalia and malaria, Gowland and Western (2012) analyzed the distribution of cribra orbitalia and enamel hypoplasia in Anglo-Saxon burials in relation to geographic variables and historically recorded distribution patterns of indigenous malaria and the habitat of its mosquito vector Anopheles atroparvus. Their modeling showed a correlation between cribra orbitalia and geography, as well as a spatial correlation between cribra orbitalia and historically recorded evidence for malaria and its mosquito vector (Gowland and Western 2012). They also found no such correlations with enamel hypoplasia, indicating a specific cause for acquired anemia rather than generalized poor health (Gowland and Western 2012). This study demonstrates how incorporating multiple lines of evidence (both direct and indirect) from a range of disciplines can help to elucidate the etiological causes of nonspecific indicators of infection and, therefore, understand the prevalence, distribution, and transmission pathways of diseases.
What these examples demonstrate is that the story of zoonotic infections in human populations is long and complex. As Pearce-Duvet (2007) points out, ecological change and anthropogenic modification of the environment are likely key factors behind disease transmission, with agriculture being just one (although important) instance that changed the transmission ecology and increased the success of preexisting pathogens (see also Vlok et al. 2021). Bioarchaeology can contribute to a more nuanced understanding of past, contemporary, and future epidemics by providing time depth to the understanding of disease dynamics and the social, biological, and environmental circumstances that give rise to epidemics (DeWitte 2016:71; Larsen 2018). To do so, however, biases and limitations in research focus and sampling need to be recognized and, where possible, overcome. In particular, presumptions of the types of human-animal interactions that may have resulted in disease transmission need to continue to be superseded by more nuanced interrogation of context-specific relationships between animals (including humans), pathogens, and the environment.
Complexity and Human-Animal Interactions: Three Case Studies
To emphasize that point, we have identified three contemporary case studies based on our own experiences that demonstrate the complex social, economic, and ecological relationships that can exist between humans and animals.
Pigs and ancestors in Papua New Guinea
Anyone who has done research in New Guinea will agree on the central importance of pigs in indigenous cosmologies, social relations, and diets. At the same time, anyone who has done research in New Guinea will also agree on the difficulty of generalizing across the societies in the most culturally diverse area of the world. The approximately 8 million people of Papua New Guinea (PNG), the independent country consisting of the eastern half of the island and nearby offshore islands, speak more than 700 distinct, mutually unintelligible languages, and there is a similar degree of cultural diversity. In West Papua, approximately 2 million people speak in excess of 200 distinct languages (Foley 2000). This cultural diversity is also reflected in the large variety of kinds of relationships between people and pigs, as well as between humans and other kinds of animals.
According to Steensberg (1980:111), pigs may have been in New Guinea for 10,000 years and were almost certainly introduced by humans. As Steensberg notes, “It is tempting to suggest that in this part of the world the breeding of pigs and the cultivation of plants were mutually related from the very beginning, because man [sic] and pigs, have similar systems of digestion, and shared the same kind of food.” In terms of diet, the relationship between pigs and humans depended on pigs’ interest in human food and people’s interest in having a source of meat. More recently, O’Connor et al. (2011) have argued for a much more recent introduction of pigs to New Guinea, perhaps as recently as 3,000 years ago. Their argument that labels, such as “Neolithic” as a shorthand for a package of traits, should be abandoned in favor of “understanding and describing the development of local economic practices and their relationships, innovations and transformations they entail” (2011:21) is consistent with the general argument of this paper.
The importance of the relationship between pigs and humans can be seen in the amount of food grown to support pig herds, especially in the highlands of New Guinea. Bourke and Vlassak (2004:vi) estimated that food production in the highland provinces of Papua New Guinea is 47% greater than what would be required to support the human population and they estimate that one-third of all sweet potato tubers grown in those provinces are fed to pigs (cf. Hide 2003). In other words, agricultural systems in New Guinea (which has some of the oldest agricultural systems in the world) are complex ecosystems that support more than human populations.
The two New Guinea societies with which one of the authors (Busse) is most familiar—Boazi-speaking peoples who live along the Fly River and the shores of Lake Murray in Papua New Guinea’s Western Province (Busse 1987, 1991, 2005), and Alekano-speaking people in and around the town of Goroka in Eastern Highlands Province (Busse 2019)—demonstrate the difficulty of generalizing in a region of such cultural diversity.
Approximately 2,500 Boazi speakers live in small villages and scattered homesteads in the swamps and savannahs on either side of the Fly River and along the northern shores of Lake Murray. They are primarily hunters, fisher folk, sago makers, and gatherers, and do not keep domesticated pigs. However, they do hunt wild pigs, along with cassowary, deer, and wallabies, primarily with bows and arrows, but also with shotguns, on the open savannahs and in the swamp forests in the Fly River floodplain. These statements suggest another difficulty with generalization. Categories such as “hunters and gatherers,” and “wild” and “domesticated” pigs, reflect the categories and concerns of Euro-Americans rather than those of Boazi speakers. This is true of cultivation practices as well; for example, while Boazi only very rarely plant sago palms, they manage their extensive sago swamps by chopping down some juvenile sago palms so that other sago palms can grow better. Similarly, while they do not keep domesticated pigs, there are wild pigs that live around the edges of some sago swamps that women feed when they go to make sago. Boazi speakers do not distinguish linguistically between pigs that are and are not fed in this way, although they do say that pigs that are not fed by humans taste better than pigs that are fed. While, at least at the time that Busse did his main field research, Boazi speakers did not talk about the transmission of diseases from pigs to humans, the possibility of disease transfer is affected by the population density and degree of feeding and also gendered patterns of behavior rather than a domestic/wild distinction.
Pigs are totem animals for members of the Basikwin clan among Boazi speakers. Basikwin is a compound word consisting of basik, meaning “pig,” and the suffix -kwin, meaning “clan.” While there are no prohibitions on killing or eating one’s own totem animal or the totems of other people, Busse was told that a widow who really loved her husband would refrain from eating his totem animal. A few people also told him that the members of a totemic group are descended from their group’s totem animal, but this is not a universally held belief. The members of a totemic group are, however, symbolically associated with their totem animals, and disrespect shown to a totem animal can be taken as disrespect toward the people for whom the animal is their totem. Such disrespect can result in violence against the person showing disrespect. These symbolic relationships help shape human-animal interactions.
Throughout the Highlands of Papua New Guinea, pigs were, and to a large degree are, the most significant form of wealth. Even today, cash has not completely replaced pigs as a necessary form of wealth in significant public exchanges such as bridewealth and compensation. Among Alekano speakers in and around Goroka, pigs continue to be a significant form of wealth, and they are raised with an eye toward future exchanges. In communities close to Goroka town, raising pigs has become a business, and when pigs are needed for public exchanges they are often bought from commercial piggeries. Nonetheless, the symbolic importance of pigs continues. At a bridewealth presentation on the northern edge of Goroka town in which Busse participated in 2015, pigs that had been raised in pens by the groom’s extended family were presented at the end of an elaborate presentation of garden food and money to the bride’s kin, who were from a much more rural area in Eastern Highlands Province (Fig. 2). In addition to representing considerable wealth, the way in which the pigs were presented was intended to overwhelm the recipients with the power and wealth of the groom’s family. The value of pigs in these exchanges shapes husbandry practices as well as the potential movement of diseased animal.1
These and other examples provide insights into the complex and diverse ways people conceptualize their relationships with pigs (see also Sillitoe 2003).2 Understanding the multiple and complex relationships between humans and pigs—ecological, economic, social, and symbolic—in New Guinea is critical for understanding the role of pigs in the spread of zoonotic diseases, a point also made by Dwyer (2006). Simple exogenous categories (e.g., wild and domesticated) are inadequate and potentially misleading in trying to understand the role of pigs (and other animals) in disease transmission. What matters are the social relationships between pigs, between humans, and between pigs and humans. Those relationships are complex, multiple, and vary both cross-culturally and through time. How pigs are raised, managed, understood, and used by humans is not the same in different parts of New Guinea or in the same community in different historical periods. The movement of pigs, through exchange, between different groups varies considerably in different parts of New Guinea. As Dwyer (2006:S172) has written, “It is neither the pigs as such, nor people as such, that provide the context within which certain parasites may flourish. To the extent that the relationship varies … so too the expression of those diseases, in either pigs or in people, will itself vary.” He goes on to conclude that, although the tremendous environmental and cultural diversity found on the island of New Guinea makes this point particularly salient there, the fine-grained details of relationships among animals, people, and disease (what he calls “the relational context”) is critical everywhere. This includes a long-term evolutionary perspective, since, despite the close relationship between humans and pigs, health in Papua New Guinea surveys suggests relatively few species of zoonotic parasites (e.g., taenia, trichonellosis) affecting humans, although important bacterial zoonoses (pigbel, Japanese encephalitis) are shared with pigs (Barnish and Ashford 1989). The reason seems to be evolutionary—both the long history of pig-human interaction and the lack of local “wild” reservoirs (Owen 2005). In a modern context, this has not protected Papua New Guinea from African swine fever and other introduced zoonoses (The Pig Site 2020). In understanding zoonotic disease and its transfer, time, space, and cultural diversity are key aspects.
Figure 2. A pig feast near Goroka in 2014. (Photo by M. Busse.)
The feral and the national herd: Bovine TB in New Zealand
In a contemporary agricultural setting, these same complexities appear although in a very different guise. Every year in New Zealand (NZ) a small number of people (three in 2016) contract M. bovis. In 2016, this comprised 1.3% of all laboratory confirmed cases of tuberculosis among humans in NZ, reflecting drops in the overall TB rate in humans and cattle (The Institute of Environmental Science and Research 2019). Yet there are three points of interest about this figure: bovine TB features largely in newspaper coverage of tuberculosis, M. bovis is still present in New Zealand when Australia (its nearest neighbor), with a similar history of compulsory herd testing, is heading to TB-free status, and the diseased animals are very differently categorized. This is a case study of the complexity of zoonotic disease ecology, the impact of history, and the way in which those histories come up against human classifications of particular animal species. It demonstrates that it is not just the actions of people and animals but the human perceptions of those animals that frame understandings of disease and ultimately transmission dynamics.
The dynamics of transmission and infection of M. bovis in animals is remarkably similar to M. tuberculosis in humans. The same pattern of infection, latency, and active disease occurs in cattle (Hancox 2003). The disease is primarily respiratory, so managed herds in close contact are an effective environment for M. bovis transmission. While in contemporary management, animals with active disease are slaughtered, TB-free herds can be subject to “breakdowns” (an episode of disease through the herd) due to an older animal developing active disease from a latent state. Transmission to humans traditionally occurred through the drinking of contaminated milk or in abattoirs (through contact with infected carcasses).
So why does it persist? Bovine TB has been controlled effectively in many countries around the world but the problem in NZ (a problem it shares with the UK and Ireland) is the existence of significant wild reservoirs of infection. In New Zealand, the major vector identified is the brushtail possum (Trichosurus vulpecula).
Possums were identified as a vector in 1971/1972. TB rates among possums are between 1% and 10%, with disease often in highly spatially aggregated areas (Ryan et al. 2006). TB is extremely effective among possums, causing death within an average of 4.7 months. Sick animals become debilitated, unable to climb, wander around in daylight, and hence are accessible to curious cattle who have been seen to mouth and sniff at the diseased animals.
However, this picture is incomplete. Possums are an import from Australia. The first successful introduction was in 1858 at the behest of acclimatization societies who saw New Zealand’s own native fauna as largely doomed and who were interested for a range of sentimental and economic interests in the importation of exotic species (Clout and Ericksen 2000). It was hoped that possums would form the basis of a successful fur trading industry. Up until 1926, there were 127 recorded liberations of possums by acclimatization societies and government agencies (most of NZ bred possums). The damage that possums were doing to NZ’s native forests was not formally taken into account until 1947 when all they were recognized as pests and poisoning was legalized.
Possums were unlikely invaders; they have a low intrinsic rate of increase but they do have broad environmental tolerance and are opportunistic feeders. The lack of competitors and parasites in NZ lead to their success, although the multiple introductions and releases were probably some of the most important factors since the chance of demographic collapse was overwhelmed and they served to ensure the genetic diversity of the species (Clout and Ericksen 2000).
Yet possums do not naturally carry TB. NZ possums caught bovine TB from those other great imported animals—cattle and deer and most probably from their carrion (Price-Carter et al. 2018). From this perspective, possums are the victim rather than the vector. What is also missing from the picture is all the other vectors of bovine TB: ferrets, feral pigs, cats, stoats, and deer. Deer in particular can be true maintenance hosts when there is sufficient contact between infected and noninfected animals. While possums may be important in the maintenance and expansion of areas known to be infected, the establishment of completely new areas of infection may be the result of other factors such as the movement of infected deer (Ryan and Livingstone 2000).
The final missing piece of the puzzle lies with the farming industry itself. Changes in agricultural practices have made the spread of TB more likely. First is the movement of cattle (Price-Carter et al. 2018; Ryan and Livingstone 2000). For example, in the Waikato region of the North Island, one study demonstrates that only 10% of dairy herds were closed; most farmers move cattle between properties, particularly when young (Ryan and Livingstone 2000).
Second is the change in herd size and expansion into new areas. The average dairy herd size in 2017 was 431 but in some areas, average herd size was as high as 800 animals (DairyNZ 2018). TB outbreaks are more likely in such large groupings both because of the movement of animals between herds but also potentially because of stress. Furthermore, farming on pasture forest margins as dairying expands allows cattle and possums to mix most freely (Coleman 1988).
The ecological picture is complex. There are multiple species involved in the disease cycle and transmission itself is along multiple routes (respiratory and oral), involving the actions of animals (diseased possums coming down to the ground during the day, curious cattle nosing sick or dead possums) and the way in which humans interact with species (not just through infected food products but close handling of animals through milking or in abattoirs), and all in a context created by human translocation of animal species and the intensification of agriculture. However, in coverage of bovine TB, all of these translocated species are labelled: possums as feral, deer as somewhat ambivalent, and cattle as the “national herd,” an integral part of New Zealand’s economy (Littleton and Park 2009). These symbolic categories influence what attention is paid in terms of prevention (possums are frequently exterminated but deer are less subject to culls, while the expansion of dairy herds and cattle movement is part of acceptable agricultural practice).
The persistence of bovine TB in New Zealand reveals how disease travels along particular lines not simply because particular animals are present, but because of the actions of those animals and the niches constructed by humans that place animals into contact with each other. It is not enough to have the host, pathogen, and environment—it is the nature of the linkages that determines whether disease eventuates and how severe that disease will be. The New Zealand case study is also a reminder of animal translocation and its significance in many historical societies. However, there are also the less dramatic movements of animals due to trade, the movement of breeding stock, and transhumance (Mashkour et al. 2005). New animals introduce new pathogens, and they can encounter new pathogens. But how much any of this is recognized and acted upon is affected by how humans perceive and categorize the animals with which they interact.
The human and nonhuman primate interface: Dynamics of disease transmission
While the two case studies so far presented demonstrate how “domestic” and “wild” might not be closed categories and how humans manipulate and create conditions for disease transmission, the time depth of human-animal connections is often tied to the notion of domestication. Yet, across cultural, historical, and ecological contexts, primates and humans share an extended coexistence. Evidence of such coexistence is temporally deep and geographically broad. In Gabon, West Africa, three ape genera (Homo, Pan, and Gorilla) shared niche space and have likely competed for plant foods for approximately 60,000 years (Tutin and Oslisly 1995). Findings from Niah Cave, Sarawak (in northwest Borneo), demonstrate the robust hunting behavior of humans upon an array of nonhuman primate species (a mere subset of the overall bone assemblage) during the terminal Pleistocene (25,000–40,000 years ago) (Harrison 1966; Piper and Rabett 2009; Sponsel et al. 2002). More recently, human predation likely contributed to the extinction of Madagascar’s giant, subfossil lemur species (Paleopropithecus spp.) (Perez et al. 2005). In contemporary Brazil, the indigenous Awá-Guajá maintain a complicated set of relationships with the endemic red-handed howler monkeys (Alouatta belzebul) whereby the primates are integral to both the Awá-Guajá’s kinship system and diet (Cormier 2002). From past to present, the trajectories of most nonhuman primate populations are determined, either directly or indirectly, by humankind’s alterations and actions (Behie et al. 2019).
Contemporary humans construct and occupy increasingly interconnected landscapes that include cities, farms, and forests. Our primate kin are routinely displaced from their habitats, hunted for meat, captured for trade, and enlisted as unwitting participants in our research and conservation interventions. Habituated animals are vulnerable to human hunting (in the absence of sustained researcher presence), and the cutting of trails and other research activities can alter delicate ecological systems (Malone et al. 2010). At the same time, the presence of researchers and/or ecotourists can expose nonhuman primates to human diseases. In fact, multiple disease outbreaks in ape populations have been linked to interactions with humans (Köndgen et al. 2008; Travis et al. 2008). Of course, given our biological similarity, pathogen transmission is bidirectional. That is, humans are susceptible to contracting diseases from nonhuman primates (anthropozoonotic transmission). Immeasurable human suffering stems from the ebolaviruses (Filoviridae), pathogens which can cause severe hemorrhagic fever and high mortality (40%–90%) in humans and other animals, including decimating outbreaks in chimpanzee and gorilla populations (Walsh et al. 2003). Additionally, the form of human immunodeficiency virus-1, the virus that caused the AIDS pandemic, resulted from the “spillover” (nonhuman animal to human transmission via contact with the reservoir host) and mutation of a chimpanzee immunodeficiency virus to humans (Gao et al. 1999; Gilardi et al. 2015). Beyond these highly publicized examples, anthropozoonoses also include retroviruses, intestinal parasites, polio, TB, and anthrax. Indeed, the current pandemic and outbreak of COVID-19 as well as SARS, MERS, and Nipah virus are also examples of anthropozoonotic spillover events (Nieuwland et al. 2022).
Microbial emergence events and the concomitant risk that infectious agents pose for human populations is related to both the level of microbial diversity (e.g., high in lowland tropical forests) and behaviors that facilitate contact between novel microbes and their prospective hosts (Wolfe et al. 2000). Incursions into, and modifications of, tropical forests as well as hunting, butchering, and pet keeping of nonhuman primates are among the riskier of such behaviors. However, comprehensive analyses of human and nonhuman primate interfaces reveal their complexity, particularly with respect to emergent diseases (Knauf and Jones-Engel 2020). A prominent example can be found in Bali, Indonesia. Balinese people and long-tailed macaques (Macaca fascicularis) live in sympatry, and have done so for over a thousand years. In this time, macaques and Balinese people have together developed a mutual ecology where lives and livelihoods are deeply entwined (Fuentes 2010; Wheatley 1999). The more recent surge of tourism in Bali provides an additional layer of relationships. The Balinese people/macaque/tourist interface incorporates dietary, economic, parasitological, religious, behavioral, political, and geographical elements (Fuentes 2006; Jones-Engel et al. 2005). The human-macaque interface in Bali is characterized by behavioral and ecological interactions embedded in a cocreated history, including: a) macaque populations that congregate at Hindu temple sites where they achieve a sacred, protected status, which partially underpins population growth; b) a tourism industry that facilitates the existences of the politically and economically driven tourist-macaque interface; c) the livelihoods of Balinese people that are financially supported by tourist-macaque interactions; d) macaque diets being modified by tourist provisioning; and e) belief systems that influence the mutual ecologies of the various actants at these sites (Fuentes 2010). Additionally, crop-raiding macaques are frequently targeted and killed by farmers outside of temple complexes. Moreover, macaques are the most frequently observed nonhuman primate species in the longstanding live animal trade, and their relative abundance has increased over time (Malone et al. 2003; Nijman et al. 2017) (Fig. 3). Given the complexity of this interface, and the associated risk of novel microbial emergence and transmission (Lane-deGraaf et al. 2014), a holistic lens that illuminates the interplay between biophysical and social elements is required (Malone and Ovenden 2017).
Figure 3. Macaques crated as part of the pet trade in Indonesia (2001). (Photo by N. Malone.)
Over the course of human evolution, our lineage has navigated new ecologies and niches, including a diverse array of plants and animals. These interactions produce reciprocal effects on bodies and behavior that are at once biological, cultural, and political (Fuentes 2020). As demonstrated here in this brief overview of human–nonhuman primate interactions, a strict emphasis on human manipulation and control of other species (formal domestication) is an inadequate lens through which to view the contexts that characterize many organisms that occupy human niche space. Fuentes (2007:124) sees human actions that alter species’ ecologies (with impacts on physiology and behavior) as resulting not in “domestic species” per se, “but species that are being directly shaped by processes (domesticatory practices) resulting from human action.” This reframing enables the effective analysis of particular contexts of contact (e.g., trade networks’ live animals or wild game from source to sale; human and macaque coexistence in Asian temple sites), and facilitates mitigation strategies accordingly. Through this more holistic, anthropological framing of the human and nonhuman primate interface, pathways of pathogen transmission, and their respective risks, are identified as components of broader ecological and sociopolitical systems.
Adopting a One Health Perspective
To understand and analyze the history of zoonotic disease and its past impacts then relies upon understanding animal and human disease in the context of social and ecological systems as well as evolutionary perspectives (Fig. 4). In contemporary health research, such work increasingly engages with a One Health perspective—an integrative approach to animal-human health (Waltner-Toews 2017) that conceptualizes the health of humans, the environment, and nonhuman animals as linked (Lebov et al. 2017).
There is nothing new in the recognition of human and animal health as linked. Day (2011) identifies instances of formal linkages between human and animal disease back to early Mesopotamia. But a One Health approach adopts a holistic understanding of health beyond the purely biomedical (Zinsstag 2012), focusing on relationships between disease dynamics, environmental drivers, livelihood systems, and veterinary and public health responses (Scoones et al. 2017). Traditionally, One Health efforts have focused on surveillance and containment, particularly of zoonotic disease, but as ecosystem approaches to health have developed, researchers have urged for greater consideration of the structural drivers behind disease in linked animal and human populations (Wallace et al. 2015), as well as for attention at the local level (Rock et al. 2009). As expressed by Hinchliffe (2015:31): “there is more than one world, and likewise more than One Health … it is crucial that we demonstrate how health is patched together in practices that take account of local conditions …”
In many respects, a One Health perspective articulates with other frameworks used in the analysis of disease in archaeological settings (e.g., biocultural perspectives, multispecies approaches, agency approaches). Different research approaches and research questions emphasize particular aspects but all (to some extent):
1. Problematize the relationship between biology and culture, understanding that disease is not a natural fact but created and recognized within particular settings;
2. Assert that understanding those settings depends upon working with different temporal scales from long-term evolutionary to the short-term life cycle of organisms;
3. Recognize human and animal environments as created not just through human histories but the interaction of species with climate and physical geography which in itself can create new selective pressures (niche construction) and that beyond the creation and modification of environments, it is important to remember that such settings are partial in that different aspects of an environment are salient to humans and animal species;
4. Identify social and economic arrangements as important to understanding disease or other aspects of human-animal relationships; and
5. Acknowledge that in the complex relationships between humans, animals, and microbes, all species are actors in that through their life cycles and actions, they make a discernible difference (Law and Mol 2008), while not necessarily having the capacity of intention.
Figure 4. A model of a One Health perspective to examine zoonotic disease, adapted from Woldehanna and Zimicki (2015:Figure 1).
So what does that suggest—an agenda for bioarchaeology?
The relationship between time since domestication and the number of shared pathogens between humans and domesticates has been demonstrated (Morand et al. 2014). However, as the case studies above show, that relationship is dependent on much more than just economic relationships or extractive activities. Recent outbreaks demonstrate the importance of spillover from enzootic reservoirs into other animal and ultimately human reservoirs. Bioarchaeology has also demonstrated that complexity. For example, the complex phylogenetic relationships of the Mycobacterium tuberculosis complex (e.g., Bos et al. 2014) and the range of potential reservoirs for plague (Green 2020) point to how the emergence, spread, and impact of zoonotic infections (and ultimately the evolution of human infections) are more complex and diverse than initially suspected.
While specific disease identification in humans or animal skeletal remains is difficult and fraught with problems of specificity and preservation, an integrated perspective opens up a range of potential evidence beyond the identification of diseased individuals (Bendrey et al. 2019; One Health Archaeology Research Group 2020). The value of such interdisciplinary studies is being recognized in modeling approaches that bring together a range of specialities.
Such models allow analysis of diseases often considered invisible in the archaeological record and embracing a One Health approach can help to untangle the complex set of human-animal–environment relationships that lead to the emergence, spread, and maintenance of zoonotic infections. The use of bioarchaeological data in modeling of disease dynamics is currently somewhat limited, but combining bioarchaeological evidence (including paleogenetics and paleoproteomics) with ecological, climate, geospatial, clinical, zooarchaeological, ethnographic, and historical data among others shows great potential for contributing to our understanding of past, present, and emerging zoonotic diseases. As argued by Bendrey et al. (2019), there are multiple sources of data. These can be used as proxies for aspects of the model shown in Figure 4. While identification of disease is a first step, it is not the last. If we think about the human aspect, consideration of demography, evidence of work patterns, diet, and movement are all possible. From the animal aspect, while identifiable lesions may not be readily available, the recognizable biodiversity at a site, the demography of animals, evidence of their diet, movement, symbolic value (burial, material culture), and, at times, health, provide indications of the animal niche. Aspects of contact are visible within settlement structures and layouts, animal and human demography, evidence of work patterns for both humans and animals, and how that is structured along age and sex/gender. These data can then be analyzed within a careful consideration of climatic and environmental change over time. Using the example of tracing the impact of malaria in Bahrain (an emergent piece of research), evidence of comorbidity over time is established (e.g., Littleton 1998, 2011), but that needs to be tied to stronger evidence for malaria (including the type of malaria from paleogenomics) and human and animal migration. Stable isotopes are providing much of the evidence for husbandry and how that varies over time and by place (Smith and Littleton 2019, 2021) as well as revealing the complexity of human migration in Bahrain. Those two long-term records can then be tied to archaeological and paleoenvironmental evidence, such as settlement distribution (Larsen 1983; Olijdam 2000), ecology (Tengberg and Lombard 2001), and water levels (Sanlaville 1992), to develop a dynamic model of disease and human-animal interaction. We are not arguing that all of those aspects of human-animal interactions will always be available (and thinking about the Bahrain example, they are certainly not), but a greater emphasis going forward on interdisciplinary research using multiple forms of evidence and developing testable models has great potential.
These sorts of analyses can provide the “place-specific deep time histories, cultural infrastructure, and economic geographies” that underlie disease emergence (Wallace et al. 2015:68). By centering and exploring the complexity of human-animal interactions within a broader ecological and evolutionary history, bioarchaeologists and their collaborators can offer theoretically informed and historically contextualized studies of humans and other species.
Such local ecologies inform how human societies have shaped and been shaped by the animals and world around them (akin to niche construction). They have the potential to challenge narratives of imminent risk, of defensive responses (Bendrey and Fournié 2020), and contribute to burning contemporary questions of disease persistence and emergence. Adopting approaches that emphasize time depth, multiple scales of activity, evolutionary understandings, and a consideration of human practices and values in creating relationships with other species will make bioarchaeological contributions relevant to contemporary and future issues beyond the epidemiological transition model.
Acknowledgments
We are grateful to the University of Auckland Arts Faculty for supporting this work through the PBRF. We would also like to thank the Biological Anthropology writing group for their constructive conversations and criticisms on this manuscript. Thanks also go to the editors of Bioarchaeology International for the opportunity to submit to this issue and to the reviewers for their helpful suggestions.
References Cited
Aufderheide, Arthur C., Wilmar Salo, Michael Madden, John Streitz, Jane Buikstra, Felipe Guhl, Bernardo Arriaza, Colleen Renier, Lorentz E. Wittmers Jr., Gino Fornaciari, and Marvin Allison. 2004. A 9,000-year record of Chagas’ disease. Proceedings of the National Academy of Sciences of the United States of America 101(7):2034–2039. DOI: 10.1073/pnas.0307312101.
Baker, Oussama, Oona Y.-C. Lee, Houdini H. T. Wu, Gurdyal S. Besra, David E. Minnikin, Gareth Llewellyn, Christopher M. Williams, Frank Maixner, Niall O’Sullivan, Albert Zink, Bérénice Chamel, Rima Khawam, Eric Coqueugniot, Daniel Helmer, Françoise Le Mort, Pascale Perrin, Lionel Gourichon, Bruno Dutailly, György Pálfi, Hélène Coqueugniot, and Olivier Dutour. 2015. Human tuberculosis predates domestication in ancient Syria. Tuberculosis 95:S4–S12. DOI: 10.1016/j.tube.2015.02.001.
Barnish, Guy, and R. W. Ashford. 1989. Occasional parasitic infections of man in Papua New Guinea and Irian Jaya (New Guinea). Annals of Tropical Medicine & Parasitology 83(2):121–135. DOI: 10.1080/00034983.1989.11812320.
Barrett, Ron, and George Armelagos. 2013. An Unnatural History of Emerging Infections. Oxford University Press, Oxford.
Barrett, Ronald, Christopher W. Kuzawa, Thomas McDade, and George J. Armelagos. 1998. Emerging and re-emerging infectious diseases: The third epidemiologic transition. Annual Review of Anthropology 27(1):247–271. DOI: 10.1146/annurev.anthro.27.1.247.
Bartosiewicz, László. 2013. Shuffling Nags, Lame Ducks: The Archaeology of Animal Disease. Oxbow Books, Oxford.
Bathurst, Rhonda R., and Jodi Lynn Barta. 2004. Molecular evidence of tuberculosis induced hypertrophic osteopathy in a 16th-century Iroquoian dog. Journal of Archaeological Science 31(7):917–925. DOI: 10.1016/j.jas.2003.12.006.
Behie, Alison M., Julie A. Teichroeb, and Nicholas Malone, eds. 2019. Primate Research and Conservation in the Anthropocene. Cambridge University Press, Cambridge.
Bendrey, Robin, Joseph P. Cassidy, Guillaume Fournié, Deborah C. Merrett, Rebecca H. A. Oakes, and G. Michael Taylor. 2019. Approaching ancient disease from a One Health perspective: Interdisciplinary review for the investigation of zoonotic brucellosis. International Journal of Osteoarchaeology 30(1):99–108. DOI: 10.1002/oa.2837.
Bendrey, Robin, and Guillaume Fournié. 2020. Zoonotic brucellosis from the long view: Can the past contribute to the present? Infection Control & Hospital Epidemiology 42(4):505–506. DOI: 10.1017/ice.2020.270.
Bos, Kirsten I., Kelly M. Harkins, Alexander Herbig, Mireia Coscolla, Nico Weber, Iñaki Comas, Stephen A. Forrest, Josephine M. Bryant, Simon R. Harris, Verena J. Schuenemann, Tessa J. Campbell, Kerttu Majander, Alicia K. Wilbur, Ricardo A. Guichon, Dawnie L. Wolfe Steadman, Della Collins Cook, Stefan Niemann, Marcel A. Behr, Martin Zumarraga, Ricardo Bastida, Daniel Huson, Kay Nieselt, Douglas Young, Julian Parkhill, Jane E. Buikstra, Sebastien Gagneux, Anne C. Stone, and Johannes Krause. 2014. Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis. Nature 514(7523):494–497. DOI: 10.1038/nature13591.
Bourke, Richard Michael, and V. Vlassak. 2004. Estimates of Food Crop Production in Papua New Guinea. Australian National University, Canberra.
Brosch Roland, Stephen V. Gordon, Magali Marmiesse, Priscille Brodin, Carmen Buchrieser, Karin Eiglmeier, Thierry Garnier, Christina Gutierrez, Glyn Hewinson, Kristin Kremer, Linda M. Parsons, Alexander S. Pym, Sofia Samper, D. van Soolingen, and Stewart T. Cole. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proceedings of the National Academy of Sciences of the United States of America 99(6):3684–3689. DOI: 10.1073/pnas.052548299.
Brothwell, Donald R. 1965. The palaeopathology of the E.B.-M.B. and middle bronze age remains from Jericho (1957–1958 excavations). In Excavations at Jericho, edited by K. M. Kenyon. British School of Archaeology in Jerusalem, London, pp.685–693.
Busse, Mark. 1987. Sister Exchange Among the Wamek of the Middle Fly. PhD dissertation, University of California, San Diego.
Busse, Mark. 1991. Boazi. In Encyclopedia of World Cultures, Volume 2: Oceania, edited by Terrence E. Hays. G. K. Hall, Boston, pp. 28–31.
Busse, Mark. 2005. “We will exchange sisters until the world ends”: Inequality, marriage and gender relations in the Lake Murray-Middle Fly area, Papua New Guinea. In A Polymath Anthropologist: Essays in Honour of Ann Chowning. Research in Anthropology and Linguistics, Monograph Number 6, edited by C. Gross, H. Lyons, and D. Counts. Department of Anthropology, University of Auckland, Auckland, NZ, pp. 79–88.
Busse, Mark. 2019. Morality and the concept of the market seller among Gehamo. Oceania 89(2):205–219. DOI: 10.1002/ocea.5220.
Capasso, Luigi. 1999. Brucellosis at Herculaneum (79 AD). International Journal of Osteoarchaeology 9(5):277–288. DOI: 10.1002/(SICI)1099-1212(199909/10)9:5<277::AID-OA489>3.0.CO;2-0.
Capasso, Luigi. 2002. Bacteria in two-millennia-old cheese, and related epizoonoses in Roman populations. Journal of Infection 45(2):122–127. DOI: 10.1053/jinf.2002.0996.
Chomel, Bruno B. 2014. Zoonoses. Reference Module in Biomedical Sciences, 820–829. DOI: 10.1016/B978-0-12-801238-3.02426-0.
Clout, Mick N., and K. Ericksen. 2000. Anatomy of a disastrous success: The brushtail possum as an invasive species. In The Brushtail Possum: Biology, Impact and Management of an Introduced Marsupial, edited by T. L. Montague. Manaaki Whenua Press, Lincoln, NZ, pp. 1–9.
Coimbra, Carlos E. A., Jr. 1988. Human settlements, demographic pattern, and epidemiology in lowland Amazonia: The case of Chagas’s disease. American Anthropologist 90(1):82–97. DOI: 10.1525/aa.1988.90.1.02a00060.
Coleman, J. D. 1988. Distribution, prevalence, and epidemiology of bovine tuberculosis in brushtail possums, Trichosurus-vulpecula, in the Hohonu Range, New-Zealand. Wildlife Research 15(6):651–663. DOI: 10.1071/WR9880651.
Cormier, Loretta A. 2002. Monkey as food, monkey as child: Guajá symbolic cannibalism. In Primates Face to Face: Conservation Implications of Human-Nonhuman Primate Interconnections, edited by Agustín Fuentes and Linda D. Wolfe. Cambridge University Press, Cambridge, pp. 63–84.
DairyNZ. 2018. New Zealand Dairy Statistics 2017–18. https://www.dairynz.co.nz/publications/dairy-industry/new-zealand-dairy-statistics-2017-18. Accessed December 18, 2020.
D’Anastasio, Ruggero, T. Staniscia, M. L. Milia, Lamberto Manzoli, and Luigi Capasso. 2011. Origin, evolution and paleoepidemiology of brucellosis. Epidemiology & Infection 139(1):149–156. DOI: 10.1017/S095026881000097X.
D’Anastasio, Ruggero, Bernhard Zipfel, Jacopo Moggi-Cecchi, Roscoe Stanyon, and Luigi Capasso. 2009. Possible brucellosis in an early hominin skeleton from Sterkfontein, South Africa. PLoS ONE 4(7):e6439. DOI: 10.1371/journal.pone.0006439.
Day, Michael J. 2011. One Health: The importance of companion animal vector-borne diseases. Parasites & Vectors 4(49). DOI: 10.1186/1756-3305-4-49.
DeWitte, Sharon N. 2016. Archaeological evidence of epidemics can inform future epidemics. Annual Review of Anthropology 45:63–77. DOI: 10.1146/annurev-anthro-102215-095929.
DeWitte, Sharon N., and James W. Wood. 2008. Selectivity of Black Death mortality with respect to preexisting health. Proceedings of the National Academy of Sciences of the United States of America 105(5):1436–1441. DOI: 10.1073/pnas.0705460105.
Dwyer, Peter D. 2006. People, pigs and parasites in New Guinea: Relational contexts and epidemiological possibilities. Parasitology International 55:S167–S173. DOI: 10.1016/j.parint.2005.11.026.
Fernandes, Alexandre, Alena M. Iñiguez, Valdirene S. Lima, Sheila M. F. Mendonça de Souza, Luiz Fernando Ferreira, Ana Carolina P. Vicente, and Ana M. Jansen. 2008. Pre-Columbian Chagas disease in Brazil: Trypanosoma cruzi I in the archaeological remains of a human in Peruaçu Valley, Minas Gerais, Brazil. Memórias do Instituto Oswaldo Cruz 103(5):514–516. DOI: 10.1590/S0074-02762008000500021.
Foley, William A. 2000. The languages of New Guinea. Annual Review of Anthropology 29:357–404. DOI: 10.1146/annurev.anthro.29.1.357.
Fournié, Guillaume, Dirk U. Pfeiffer, and Robin Bendrey. 2017. Early animal farming and zoonotic disease dynamics: Modelling brucellosis transmission in Neolithic goat populations. Royal Society Open Science 4(2):e160943. DOI: 10.1098/rsos.160943.
Fuentes, Agustín. 2006. Human culture and monkey behavior: Assessing the contexts of potential pathogen transmission between macaques and humans. American Journal of Primatology 68(9):880–896. DOI: 10.1002/ajp.20295.
Fuentes, Agustín. 2007. Monkey and human interconnections: The wild, the captive, and the in-between. In Where the Wild Things Are Now: Domestication Reconsidered, edited by Rebecca Cassidy and Molly Mullin. Berg, Oxford, pp. 123–145.
Fuentes, Agustín. 2010. Naturalcultural encounters in Bali: Monkeys, temples, tourists, and ethnoprimatology. Cultural Anthropology 25(4):600–624. DOI: 10.1111/j.1548-1360.2010.01071.x.
Fuentes, Agustín. 2020. A (bio)anthropological view of the COVID-19 era midstream: Beyond the infection. Anthropology Now 12(1):24–32. DOI: 10.1080/19428200.2020.1760635.
Gao, Feng, Elizabeth Bailes, David L. Robertson, Yalu Chen, Cynthia M. Rodenburg, Scott F. Michael, Larry B. Cummins, Larry O. Arthur, Martine Peeters, George M. Shaw, Paul M. Sharp, and Beatrice H. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397(6718):436–441. DOI: 10.1038/17130.
Gilardi, Kirsten V. K., Thomas R. Gillespie, Fabian H. Leendertz, Elizabeth J. Macfie, Dominic A. Travis, Christopher A. Whittier, and Elizabeth A. Williamson. 2015. Best Practice Guidelines for Health Monitoring and Disease Control in Great Ape Populations. IUCN SSC Primate Specialist Group, Gland, CH.
Gowland, Rebecca L., and A. Gaynor Western. 2012. Morbidity in the marshes: Using spatial epidemiology to investigate skeletal evidence for malaria in Anglo-Saxon England (AD 410–1050). American Journal of Physical Anthropology 147(2):301–311. DOI: 10.1002/ajpa.21648.
Green, Monica H. 2020. The four black deaths. The American Historical Review 125(5):1601–1631. DOI: 10.1093/ahr/rhaa511.
Hancox, M. 2003. Latency and the control of bovine TB in man and other animals. Respiratory Medicine 97(9):1075–1077. DOI: 10.1016/s0954-6111(03)00135-5.
Harrisson, Ted. 1966. Lobang Angus, a frequentation cave at Niah—I. Sarawak Museum Journal 14(28–29 New Series):217–223.
Hershkovitz, Israel, Helen D. Donoghue, David E. Minnikin, Gurdyal S. Besra, Oona Y.-C. Lee, Angela M. Gernaey, Ehud Galili, Vered Eshed, Charles L. Greenblatt, Eshetu Lemma, Gila Kahila Bar-Gal, and Mark Spigelman. 2008. Detection and molecular characterisation of 9000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the eastern Mediterranean. PLoS ONE 3(10):e3426. DOI: 10.1371/journal.pone.0003426.
Hide, Robin. 2003. Pig Husbandry in New Guinea: A Literature Review and Bibliography. Australian Centre for International Agricultural Research, Canberra.
Hinchliffe, Steve. 2015. More than one world, more than One Health: Re-configuring interspecies health. Social Science & Medicine 129:28–35. DOI: 10.1016/j.socscimed.2014.07.007.
Jones, Christine. 2019. Brucellosis in an adult female from Fate Bell Rock Shelter, Lower Pecos, Texas (4000–1300 BP). International Journal of Paleopathology 24:252–264. DOI: 10.1016/j.ijpp.2019.01.005.
Jones-Engel, Lisa, Gregory A. Engel, and Michael A. Schillaci. 2005. An ethnoprimatological assessment of disease transmission among humans and wild and pet macaques on the Indonesian island of Sulawesi. In Commensalism and Conflict: The Human-Primate Interface, edited by James D. Patterson and Janette Wallis. American Society of Primatologists, Norman, OK, pp. 196–221.
Klauder, Joseph V. 1958. Interrelations of human and veterinary medicine—discussion of some aspects of comparative dermatology. New England Journal of Medicine 258(4):170–177. DOI: 10.1056/NEJM195801232580405.
Knauf, Sascha, and Lisa Jones-Engel, eds. 2020. Neglected Diseases in Monkeys: From the Monkey-Human Interface to One Health. Springer International Publishing, New York.
Köndgen, Sophie, Hjalmar Kühl, Paul K. N’Goran, Peter D. Walsh, Svenja Schenk, Nancy Ernst, Roman Biek, Pierre Formenty, Kerstin Mätz-Rensing, Brunhilde Schweiger, Sandra Junglen, Heinz Ellerbrok, Andreas Nitsche, Thomas Briese, W. Ian Lipkin, Georg Pauli, Christophe Boesch, and Fabian H. Leendertz. 2008. Pandemic human viruses cause decline of endangered great apes. Current Biology 18(4):260–264. DOI: 10.1016/j.cub.2008.01.012.
Lane‐deGraaf, Kelly E., I. G. A. Arta Putra, I. Nengah Wandia, Aida Rompis, Hope Hollocher, and Agustín Fuentes. 2014. Human behavior and opportunities for parasite transmission in communities surrounding long‐tailed macaque populations in Bali, Indonesia. American Journal of Primatology 76(2):159–167. DOI: 10.1002/ajp.22218.
Larsen, Clark Spencer. 2018. The bioarchaeology of health crisis: Infectious disease in the past. Annual Review of Anthropology 47:295–313. DOI: 10.1146/annurev-anthro-102116-041441.
Larsen, Curtis E. 1983. Life and Land Use on the Bahrain Islands: The Geoarchaeology of an Ancient Society. University of Chicago Press, Chicago.
Law, John, and Annemarie Mol. 2008. The actor-enacted: Cumbrian sheep in 2001. In Material Agency: Towards a Non-Anthropocentric Approach, edited by Carl Knappett and Lambros Malafouris. Springer, New York, pp. 57–77.
Lebov, Jill, Khara Grieger, Donna Womack, Daniel Zaccaro, Nedra Whitehead, Barbara Kowalcyk, and Pia D. M. MacDonald. 2017. A framework for One Health research. One Health 3:44–50. DOI: 10.1016/j.onehlt.2017.03.004.
Littleton, Judith. 1998. Skeletons and Social Composition: Bahrain 250 BC–250 AD. British Archaeological Reports, Oxford.
Littleton, Judith. 2011. Moving from the canary in the coalmine: Modeling childhood in Bahrain. In Social Bioarcheology, edited by Sabrina C. Agarwal and Bonnie A. Glencross. Wiley-Blackwell, Chichester, UK, pp. 361–389.
Littleton, Judith, and Julie Park. 2009. Bovine tuberculosis: The feral and the national herd. Paper presented at the 50th Anniversary Conference of the Society for Medical Anthropologists, New Haven, MA.
MacKinnon, Michael. 2010. “Sick as a dog”: Zooarchaeological evidence for pet dog health and welfare in the Roman world. World Archaeology 42(2):290–309. DOI: 10.1080/00438241003673011.
Malone, Nicholas M., Agustín Fuentes, Asep R. Purnama, and Indra M. W. Adi Putra. 2003. Displaced hylobatids: Biological, cultural, and economic aspects of the primate trade in Jawa and Bali, Indonesia. Tropical Biodiversity 8(1):41–49.
Malone, Nicholas M., Agustín Fuentes, and Frances J. White. 2010. Ethics commentary: Subjects of knowledge and control in field primatology. American Journal of Primatology 72(9):779–784. DOI: 10.1002/ajp.20840.
Malone, Nicholas, and Kathryn Ovenden. 2017. Natureculture. In The International Encyclopedia of Primatology, edited by Agustín Fuentes. John Wiley & Sons, Hoboken, NJ, pp. 1–2.
Marciniak, Stephanie, D. Ann Herring, Alessandra Sperduti, Hendrik N. Poinar, and Tracy L. Prowse. 2018. A multi-faceted anthropological and genomic approach to framing Plasmodium falciparum malaria in Imperial period central-southern Italy (1st–4th c. CE). Journal of Anthropological Archaeology 49:210–224. DOI: 10.1016/j.jaa.2018.01.004.
Mashkour, Marjan, Hervé Bocherens, and Issam Moussa. 2005. Long distance movement of sheep and goats of Bakhtiari nomads tracked with intra-tooth variations of stable isotopes (13C and 18O). In Diet and Health in Past Animal Populations: Current Research and Future Directions, edited by Jessica J. Davies, M. Fabiš, I. Mainland, M. Richards, and R. Thomas. Oxbow Books, Oxford, pp. 113–124.
Maudlin, Ian, Mark Charles Eisler, and Susan Christina Welburn. 2009. Neglected and endemic zoonoses. Philosophical Transactions of the Royal Society B: Biological Sciences 364(1530):2777–2787. DOI: 10.1098/rstb.2009.0067.
McDonald, S. K., Elizabeth A. Matisoo-Smith, Hallie R. Buckley, Richard K. Walter, Htin Lin Aung, Catherine J. Collins, Gregory M. Cook, Olga Kardailsky, Johannes Krause, and Michael Knapp. 2020. “TB or not TB”: The conundrum of pre-European contact tuberculosis in the Pacific. Philosophical Transactions of the Royal Society B: Biological Sciences 375(1812):20190583. DOI: 10.1098/rstb.2019.0583.
McNeill, William H. 1976. Plagues and Peoples. Anchor Press, New York.
Merrett, Deborah Claire. 2004. Bioarchaeology in Early Neolithic Iran: Assessment of Health Status and Subsistence Strategy. PhD dissertation, University of Manitoba, Manitoba, CA.
Morand, Serge, K. Marie McIntyre, and Matthew Baylis. 2014. Domesticated animals and human infectious diseases of zoonotic origins: Domestication time matters. Infection, Genetics and Evolution 24:76–81. DOI: 10.1016/j.meegid.2014.02.013.
Moreno, Edgardo. 2014. Retrospective and prospective perspectives on zoonotic brucellosis. Frontiers in Microbiology 5:e213. DOI: 10.3389/fmicb.2014.00213.
Murphy, Eileen M., Yury K. Chistov, Richard Hopkins, Paul Rutland, and G. Michael Taylor. 2009. Tuberculosis among Iron Age individuals from Tyva, South Siberia: Palaeopathological and biomolecular findings. Journal of Archaeological Science 36(9):2029–2038. DOI: 10.1016/j.jas.2009.05.025.
Mutolo, Michael J., Lindsey L. Jenny, Amanda R. Buszek, Todd W. Fenton, and David R. Foran. 2012. Osteological and molecular identification of brucellosis in ancient Butrint, Albania. American Journal of Physical Anthropology 147(2):254–263. DOI: 10.1002/ajpa.21643.
Nieuwland, Joachim, Hope Ferdowsian, Emily Otali, J. Hartfelt, J. B. Mulcahy, D. Goodrich, and Nicholas Malone. 2022. (Nonhuman) ape health and ethics. In State of the Apes: Health and Ape Conservation, edited by Helga Rainer, Annette Lanjouw, Steve Unwin, and Alison White. Arcus Foundation, New York.
Nijman, Vincent. 2004. Conservation of the Javan gibbon Hylobates moloch: Population estimates, local extinctions, and conservation priorities. The Raffles Bulletin of Zoology 52(1):271–280.
Nijman, Vincent, Denise Spaan, Eva Johanna Rode‐Margono, Wirdateti, and K. A. I. Nekaris. 2017. Changes in the primate trade in Indonesian wildlife markets over a 25‐year period: Fewer apes and langurs, more macaques, and slow lorises. American Journal of Primatology 79(11):e22517. DOI: 10.1002/ajp.22517.
O’Connor, Sue, Anthony Barham, Kenneth Aplin, Keith Dobney, Andrew Fairbairn, and Michael Richards. 2011. The power of paradigms: Examining the evidential basis for early to mid-Holocene pigs and pottery in Melanesia. Journal of Pacific Archaeology 2(2):1–25.
Olijdam, Eric. 2000. Towards a more balanced assessment of land use on Bahrain during the City II period. Proceedings of the Seminar for Arabian Studies 30:157–163.
One Health Archaeology Research Group. 2020. One Health Archaeology Research Group. https://www.ed.ac.uk/history-classicsarchaeology/research/research-groups/one-health-archaeology. Accessed March 23, 2021.
Ortner, Donald J., and Bruno Frohlich. 2007. The EB 1A tombs and burials of Bâb edh-Dhrâ, Jordan: A bioarchaeological perspective on the people. International Journal of Osteoarchaeology 17(4):358–368. DOI: 10.1002/oa.907.
Owen, Ifor L. 2005. Parasitic zoonoses in Papua New Guinea. Journal of Helminthology 79(1):1–14. DOI: 10.1079/joh2004266.
Pearce-Duvet, Jessica M. C. 2007. The origin of human pathogens: Evaluating the role of agriculture and domestic animals in the evolution of human disease. Biological Reviews 81(3):369–382. DOI: 10.1017/S1464793106007020.
Perez, Ventura R., Laurie R. Godfrey, Malgosia Nowak-Kemp, David A. Burney, Jonah Ratsimbazafy, and Natalia Vasey. 2005. Evidence of early butchery of giant lemurs in Madagascar. Journal of Human Evolution 49(6):722–742. DOI: 10.1016/j.jhevol.2005.08.004.
Piper, Philip J., and Ryan J. Rabett. 2009. Hunting in a tropical rainforest: Evidence from the Terminal Pleistocene at Lobang Hangus, Niah Caves, Sarawak. International Journal of Osteoarchaeology 19(4):551–565. DOI: 10.1002/oa.1046.
Price-Carter, Marian, Rudiger Brauning, Geoffrey W. de Lisle, Paul Livingstone, Mark Neill, Jane Sinclair, Brent Paterson, Gillian Atkinson, Garry Knowles, Kevin Crews, Joseph Crispell, Rowland Kao, Suelee Robbe-Austerman, Tod Stuber, Julian Parkhill, James Wood, Simon Harris, and Desmond M. Collins. 2018. Whole genome sequencing for determining the source of Mycobacterium bovis infections in livestock herds and wildlife in New Zealand. Frontiers in Veterinary Science 5:272. DOI: 10.3389/fvets.2018.00272.
Rashidi, Jennifer Susan. 2011. Paleoepidemiology of Mesopotamia and the Ancient Near East: The Impact of Zoonotic Diseases and Population Demographics on Infectious Disease Patterns. PhD dissertation, University of California, Los Angeles.
Reinhard, Karl J., and Adauto Araújo. 2015. Prehistoric earth oven facilities and the pathoecology of Chagas disease in the Lower Pecos Canyonlands. Journal of Archaeological Science 53:227–234. DOI: 10.1016/j.jas.2014.09.022.
Rock, Melanie, Bonnie J. Buntain, Jennifer M. Hatfield, and Benedikt Hallgrímsson. 2009. Animal-human connections, “One Health,” and the syndemic approach to prevention. Social Science & Medicine 68(6):991–995. DOI: 10.1016/j.socscimed.2008.12.047.
Ryan, Terry J., and Paul Livingstone. 2000. Risk analysis: Movement of cattle from tuberculosis infected herds. Surveillance 27(1):8–10.
Ryan, Terry J., Paul G. Livingstone, David S. L. Ramsey, Geoffrey W. de Lisle, Graham Nugent, Desmond M. Collins, and Bryce M. Buddle. 2006. Advances in understanding disease epidemiology and implications for control and eradication of tuberculosis in livestock: The experience from New Zealand. Veterinary Microbiology 112(2–4):211–219. DOI: 10.1016/j.vetmic.2005.11.025.
Sanlaville, Paul. 1992. Changements climatiques dans la péninsule Arabique durant le Pléistocène supérieur et l’Holocène. Paléorient 18(1):5–26.
Scoones, Ian, Kate Jones, Giovanni Lo Iacono, David W. Redding, Annie Wilkinson, and James L. N. Wood. 2017. Integrative modelling for One Health: Pattern, process and participation. Philosophical Transactions of the Royal Society B: Biological Sciences 372(1725):20160164. DOI: 10.1098/rstb.2016.0164.
Seetah, Krish, Desiree LaBeaud, Jochen Kumm, Elysse Grossi-Soyster, Alfred Anangwe, and Michele Barry. 2020. Archaeology and contemporary emerging zoonosis: A framework for predicting future Rift Valley fever virus outbreaks. International Journal of Osteoarchaeology 30(3):345–354. DOI: 10.1002/oa.2862.
Sillitoe, Paul. 2003. Managing Animals in New Guinea: Preying the Game in the Highlands. Routledge, London.
Smith, Caitlin B. and Judith Littleton. 2019. Dietary analysis using stable isotopes from Bahrain: Some preliminary results. Poster presented at 53rd Seminar for Arabian Studies, University of Leiden, Leiden, NL.
Smith, Caitlin B., and Littleton, Judith. 2021. Human and faunal stable carbon and nitrogen data from Qal’at al-Bahrain. Proceedings of the Seminar for Arabian Studies.
Smith-Guzmán, Nicole E. 2015. The skeletal manifestation of malaria: An epidemiological approach using documented skeletal collections. American Journal of Physical Anthropology 158(4):624–635. DOI: 10.1002/ajpa.22819.
Smith-Guzmán, Nicole E., Jerome C. Rose, and Kathleen Kuckens. 2016. Beyond the differential diagnosis: New approaches to the bioarchaeology of the Hittite plague. In New Directions in Biocultural Anthropology, edited by Molly K. Zuckerman and Debra L. Martin. John Wiley & Sons, Hoboken, NJ, pp. 295–316.
Solari, Aldo. 2011. Past and present of Chagas disease in northern Chile. Chungara, Revista de Antropología Chilena 43(2):315–322.
Sponsel, Lesley E., Nukul Ruttanadakul, and Poranee Natadecha-Sponsel. 2002. Monkey business? The conservation implications of macaque ethnoprimatology in southern Thailand. In Primates Face to Face: Conservation Implications of Human-Nonhuman Primate Interconnections, edited by Agustín Fuentes and Linda D. Wolfe. Cambridge University Press, Cambridge, pp. 288–309.
Stead, William W. 1997. The origin and erratic global spread of tuberculosis: How the past explains the present and is the key to the future. Clinics in Chest Medicine 18(1):65–77. DOI: 10.1016/s0272-5231(05)70356-7.
Stead, William W., Kathleen D. Eisenach, M. Donald Cave, Marjorie L. Beggs, Gary L. Templeton, Charles O. Thoen, and Joseph H. Bates. 1995. When did Mycobacterium tuberculosis infection first occur in the New World? An important question with public health implications. American Journal of Respiratory and Critical Care Medicine 151:1267–1268. DOI: 10.1164/ajrccm/151.4.1267.
Steensberg, Axel. 1980. New Guinea Gardens: A Study of Husbandry with Parallels in Prehistoric Europe. Academic Press, London.
Taylor, G. Michael, Eileen Murphy, Richard Hopkins, Paul Rutland, and Yuri Chistov. 2007. First report of Mycobacterium bovis DNA in human remains from the Iron Age. Microbiology 153(4):1243–1249. DOI: 10.1099/mic.0.2006/002154-0.
Taylor, Louise H., Sophia M. Latham, and Mark E. J. Woolhouse. 2001. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society B: Biological Sciences 356(1411):983–989. DOI: 10.1098/rstb.2001.0888.
Tengberg, Margareta, and Pierre Lombard. 2001. Environnement et économie végétale à Qal’at al-Bahreïn aux périodes Dilmoun et Tylos. Premiers éléments d’archéobotanique. Paléorient 27(1):167–181.
The Institute of Environmental Science and Research Ltd (ESR). 2019. Tuberculosis in New Zealand: Annual Report 2016. ESR, Porirua, NZ.
The Pig Site. 2020. African swine fever confirmed in Papua New Guinea. https://www.thepigsite.com/news/2020/04/african-swine-fever-confirmed-in-papua-new-guinea. Accessed January 12, 2021.
Thomas, Richard. 2017. The zooarchaeology of animal “care.” In Care in the Past: Archaeological and Interdisciplinary Perspectives, edited by Lindsay Powell, William Southwell-Wright, and Rebecca Gowland. Oxbow Books, Oxford, pp. 169–188.
Travis, Dominic A., Elizabeth V. Lonsdorf, Titus Mlengeya, and Jane Raphael. 2008. A science-based approach to managing disease risks for ape conservation. American Journal of Primatology 70(8):745–750. DOI: 10.1002/ajp.20566.
Tutin, Charles-Edward G., and R. Oslisly. 1995. Homo, Pan and Gorilla: Co-existence over 60,000 years at Lopé in central Gabon. Journal of Human Evolution 28(6):597–602. DOI: 10.1006/JHEV.1995.1044.
Upex, Beth, and Keith Dobney. 2011. More than just mad cows: Exploring human-animal relationships through animal paleopathology. In A Companion to Paleopathology, edited by Anne L. Grauer. Blackwell, Oxford, pp. 191–213.
Vlok, Melandri, Hallie R. Buckley, Justyna J. Miszkiewicz, Meg M. Walker, Kate Domett, Anna Willis, Hiep H. Trinh, Tran T. Minh, Mai Huong T. Nguyen, Lan Cuong Nguyen, Hiofumi Matsumura, Tianyi Wang, Huu T. Nghia, and Marc F. Oxenham. 2021. Forager and farmer evolutionary adaptations to malaria evidenced by 7000 years of thalassemia in Southeast Asia. Scientific Reports 11(1):1–15. DOI: 10.1038/s41598-021-83978-4.
Wallace, Robert G., Luke Bergmann, Richard Kock, Marius Gilbert, Lenny Hogerwerf, Rodrick Wallace, and Mollie Holmberg. 2015. The dawn of structural One Health: A new science tracking disease emergence along circuits of capital. Social Science & Medicine 129:68–77. DOI: 10.1016/j.socscimed.2014.09.047.
Walsh, Peter D., Kate A. Abernethy, Magdalena Bermejo, Rene Beyers, Pauwel de Wachter, Marc Ella Akou, Bas Huijbregts, Daniel Idiata Mambounga, Andre Kamden Toham, Annelisa M. Kilbourn, Sally A. Lahm, Stefanie Latour, Fiona Maisels, Christian Mbina, Yves Mihindou, Sosthène Ndong Obiang, Ernestine Ntsame Effa, Malcolm P. Starkey, Paul Telfer, Marc Thibault, Caroline E. G. Tutin, Lee J. T. White, and David S. Wilkie. 2003. Catastrophic ape decline in western equatorial Africa. Nature 422(6932):611–614. DOI: 10.1038/nature01566.
Waltner-Toews, David. 2017. Zoonoses, One Health and complexity: Wicked problems and constructive conflict. Philosophical Transactions of the Royal Society B: Biological Sciences 372(1725):20160171. DOI: 10.1098/rstb.2016.0171.
Wheatley, Bruce P. 1999. The Sacred Monkeys of Bali. Waveland Press, Long Grove, IL.
Woldehanna, Sara, and Susan Zimicki. 2015. An expanded One Health model: Integrating social science and One Health to inform study of the human-animal interface. Social Science & Medicine 129:87–95. DOI: 10.1016/j.socscimed.2014.10.059.
Wolfe, Nathan D., Mpoudi Ngole Eitel, Jim Gockowski, Pia K. Muchaal, Christian Nolte, A. Tassy Prosser, Judith Ndongo Torimiro, Stephan F. Weise, and Donald S. Burke. 2000. Deforestation, hunting and the ecology of microbial emergence. Global Change and Human Health 1(1):10–25. DOI: 10.1023/A:1011519513354.
Wolfe, Nathan D., Claire Panosian Dunavan, and Jared Diamond. 2007. Origins of major human infectious diseases. Nature 447(7142):279–283. DOI: 10.1038/nature05775.
Wood, James W., George R. Milner, Henry C. Harpending, and Kenneth M. Weiss. 1992. The osteological paradox: Problems of inferring prehistoric health from skeletal samples. Current Anthropology 33(4):343–358.
Wooding, Jeanette E., Sarah S. King, G. Michael Taylor, Christopher J. Knüsel, Julie M. Bond, and John Strickland Dent. 2019. Reviewing the palaeopathological evidence for bovine tuberculosis in the associated bone groups at Wetwang Slack, East Yorkshire. International Journal of Osteoarchaeology Special Issue:1–12. DOI: 10.1002/oa.2846.
Zink, Albert R., Erika Molnár, N. Notamedi, György Pálfy, Antónia Marcsik, and Andreas G. Nerlich. 2007. Molecular history of tuberculosis from ancient mummies and skeletons. International Journal of Osteoarchaeology 17(4):380–391. DOI: 10.1002/oa.909.
Zinsstag, Jakob. 2012. Convergence of Ecohealth and One Health. EcoHealth 9(4):371–373. DOI: 10.1007/s10393-013-0812-z.
1. People in and around Goroka did not mention the transmission of diseases from pigs to humans to Busse, but that was not a topic about which he specifically asked.
2. Sillitoe (2003:278–280, 297) describes in some detail the diseases that Wola people, who live in Southern Highland Province of Papua New Guinea, attribute to pigs and how they deal with those diseases. The most common ways of dealing with pig diseases is culling and quarantining the pigs, although they also treat pigs by feeding them certain medicinal leaves and using poultices for skin conditions (2003:280). Although Sillitoe does discuss disease epidemics among pigs (2003:297), and possible ways in which New Guinea Highlanders avoid them, he does not discuss transmission of diseases from pigs to humans.
aAnthropology, The University of Auckland, Auckland 1010, New Zealand
*Correspondence to: Judith Littleton, Anthropology, The University of Auckland, Private Mail Bag 92019, Auckland City, New Zealand.
e-mail: j.littleton@auckland.ac.nz